
Diabetic Chronic Kidney Disease Study
A Phase 3 Randomized Controlled Study Of Renal Autologous Cell Therapy (React) In Subjects With Type 2 Diabetes And Chronic Kidney Disease (REGEN-006)
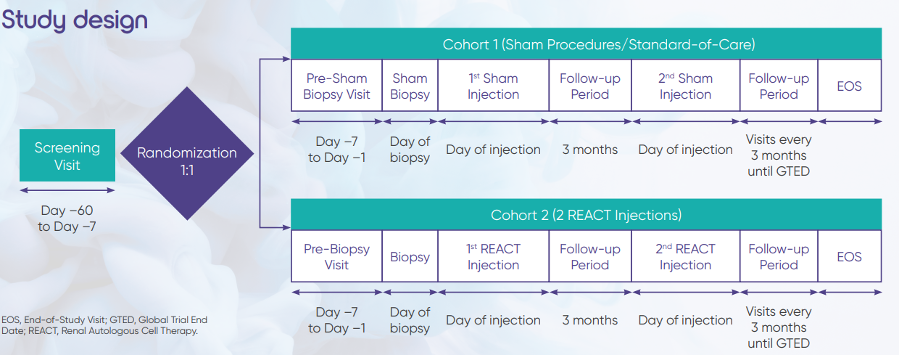
Background: REACT is an investigational cell-based advanced therapy product composed of selected renal cells naturally involved in renal repair and regeneration. 1–3 Biopsied renal cells obtained from patients are expanded ex vivo to form the active biological ingredient of the REACT product. REACT is then injected into the patient’s renal cortex using image-guided non-cutting percutaneous needle injection. This study will evaluate whether 2 REACT injections given 3 months apart into contralateral kidneys can stabilize, prevent, or delay renal function decline in patients with T2DM and CKD.
· Study eligibility will be determined.
· Assessments include, but are not limited to, physical examination, vital signs, electrocardiogram, renal imaging, blood tests, and questionnaires.
Biopsy visits
· Eligible participants will be randomized 1:1 to 1 of the 2 cohorts.
· Participants in Cohort 1 will have a scripted, simulated (fake) biopsy under conscious sedation during which no tissue will be taken. They will also undergo 2 scripted, simulated injection procedures.
· Participants in Cohort 2 will have a biopsy and receive 2 REACT injections.
· Only participants will be blinded to the study injection assignment.
· Participants will have a telephone follow-up visit 1 day after the biopsy.
Injection visits
· The first study injection will be administered in the biopsied kidney 12 weeks after the biopsy.
· Participants will have 4 follow-up study center visits for 10 weeks after the first study injection.
· The second study injection will be administered 3 months after the first study injection in the non-biopsied contralateral kidney.
· Participants will have 3 follow-up study center visits for 1 month after the second study injection.
· Participants in the simulated cohort will receive simulated (fake) injections under conscious sedation.
Long-term follow-up period
· Participants will have follow-up visits every 3 months, starting 6 months after the first study injection, until the Global Trial End Date.
Key Inclusion Criteria
- The participant is male or female, 30 to 80 years of age on the date of informed consent.
- The participant has a clinical diagnosis of T2DM in their health record.
- The participant has a clinical diagnosis of diabetic nephropathy as the underlying cause of renal disease (diagnosis does not have to be confirmed via renal biopsy) in their health record.
- The participant has a serum glycosylated hemoglobin (HbA1c) less than 10% at the Screening Visit.
- The participant has a documented clinical diagnosis of an eGFR greater than or equal to 20 and less than or equal to 50 mL/min/1.73m², not requiring renal dialysis.
- The participant has a urinary albumin-to-creatinine ratio (UACR) of greater than or equal to 300 and less than or equal to 5,000 mg/g.
Key Exclusion Criteria
- The participant has a history of type 1 diabetes mellitus.
- The participant has a history of renal transplantation.
- The participant has a mean systolic blood pressure greater than or equal to 140 mmHg and/or mean diastolic blood pressure greater than or equal to 90 mmHg at screening, across 3 measurements while seated.
- The participant has hemoglobin levels less than 10 g/dL and is not responsive to the standard medical intervention for CKD-related anemia prior to randomization.
- The patient has a bleeding disorder(s) and is maintained on any anticoagulants that cannot be discontinued for 7 days before and 7 days after the biopsy or injections.
Contact our Clinical Research Center
4141-A Duke Street
Alexandria, VA 22304-2415
Phone: 703-751-1630 (Alternate phone: 703-461-3556)
Fax: 703-461-8075
Or fill out the contact form below
